
Peter Lems is a (lung) cancer patient since 2018. He is based in the Netherlands and has become an independent patient advocate specialising in the policy of governments and their advocacy bodies on cancer drugs. Peter became a Dutch National Fellow through the EUPATI Nederland national patient educational programme in 2022. Peter is also part of the HTA4Patients project as a member of the Project Expert Panel (PEP) which is a key feedback group consisting multi-stakeholders in the Health Technology Assessment (HTA) field.
We got in touch with Peter over the summer to ask him about his story, how be become involved in the Dutch EUPATI National Platform (ENP) and about the value for patient involvement in HTA processes.
Could you share your story with us?
At the end of 2018, doctors discovered a large tumour in my lung. Research showed that there were no metastases and that my condition allowed for major lung surgery (lobectomy). Six weeks after the diagnosis and many examinations later, I was in the operating room of the hospital nearby. The operation lasted 3 hours during which the lower right lung lobe with tumour was completely removed.
I was given the opportunity for chemotherapy to eliminate any floating cancer cells, but according to my pulmonologist, that wouldn’t do much. So I refrained from doing so. Immediately after the operation, a fistula developed between lung bronchi and the space between the membranes lining the lungs (bronchopleural fistula) which allowed bacteria to accumulate. This is one of the known complications of lung (cancer) surgery that is relatively rare (20 patients per year in the Netherlands).
For this dangerous condition there are no treatment protocols together with the surgeons we had to find the best solution to combat this rare condition. The aim of the treatment was to close the fistula and the treatment could take at least a year. After several admissions, experimental treatments in different hospitals, draining, many outpatient visits, a lot of blood samples, imaging tests, surgeries and daily cleaning of the wound, I finally got rid of this problem.
During this time when a lot was happening, I got to know the hospital care from its best side. Committed professionals who want to make the best of it. But because a lot happens in a short time, you as a patient also have to be alert. I also learned that you have to coordinate your own process as well because no one else does that for you. The patient is the conductor of his/her own care.
How did you become involved in the EUPATI National Platform in the Netherlands?
Soon after my lung surgery, I became active in various cancer organisations in the Netherlands. In The KWF Dutch Cancer Society, the largest donor of cancer research in the Netherlands, I became a member of the Patient Advisory Committee that co-decides which of the many requested studies are subsidized.
I became a moderator at Kanker.NL which is an online platform for information about cancer which exchanges information and experiences between patients and their family members. I moderated the group “exercise, sports and physical recovery” because as an active athlete I know how important sports and exercise is. And how difficult it often is to exercise as a cancer patient due to interruptions in routine exercise.
I also became, for a short time, active in the patient organisation Lung Cancer Netherlands and there I noticed that the policy on cancer drugs was left to an umbrella organisation and there was relatively little knowledge among the patient organisations themselves. Here I met someone who had just completed the EUPATI training in the Netherlands and was enthusiastically told about it. I registered for the EUPATI course 2021-2022 and after acceptance I participated with great pleasure. The Corona pandemic had just ended, therefore a large part of the course could take place in-person.
Can you describe your first-hand experiences of being around the table and working together with various stakeholders?
I have worked most of my professional life in hospitals as a consultant, interim manager, manager and entrepreneur. A hospital is an extremely complex organisation with a large number of stakeholders who all have their own responsibilities and expertise that must be closely aligned. Here the art of balancing prevails.
The collaboration involves coordinating different timeframes. Doctors work with minutes, nurses with quarters, department heads with days and weeks, unit managers with months and Boards of Directors with years. The art is to take all that into account and coordinate these timeframes.
Another important element to consider is the coordination between the financial interests of a hospital and the best patient care. The housekeeping book must be correct and patients must receive the best care.
The third area of co-operational focus is the internal hospital affairs and the external environment. A hospital is part of the identity of a local community. If the hospital disappears or is given a different function due to economies of scale, this leads to strong reactions in the local community
Why is Health Technology Assessment (HTA) such an important topic?
Although the term HTA suggests that organisations that deal with this topic are looking at all kinds of medical technologies, in practice it is often about medicines. HTA organisations assess EMA-approved drugs for their efficacy and cost compared to existing drugs. National HTA organisations advise governments which medicines are eligible for reimbursement from the basic package. In view of the large supply of new medicines, choices have to be made.
Based on the research provided by pharmaceutical companies, the EMA looks at the efficacy of a drug and the risks. If the benefits outweigh the risks, the drug may be licensed to market. The medicines authority does not pay attention to other medicines that have the same objective, nor to the costs for which the new medicine is placed on the market.
HTA organisations form the link between the EMA and the national and/or regional organisations that will or will not include the medicines in their package of care. A negative review from a HTA organisation usually leads to the drug not being included in the basic package. Sometimes a drug is decided negatively because it has no added value over an existing drug, but often the new drug is equivalent or better but more expensive.
Countries seek a balance between the potential added value of a new medicine and the cost. There is support among the population to pay more for medicines that are more effective than existing ones. Implicitly or explicitly, these HTA organisations work with the financial value of a good quality year of life (qaly). In the Netherlands, this was once set at a maximum of €80,000. If a new drug far exceeds that amount, the Dutch HTA organisation will usually advise not to include it (yet) in the basic package and to negotiate the price of the drug.
For cancer drugs, from 2025 onwards, an assessment of the cost-effectiveness of a new drug will be taken at European level. Hence the HTA4Patients project. It is then up to the Member States to determine whether that assessment leads to inclusion in the basic package or not.
What is the added value for patient involvement in HTA processes?
The advice from HTA organisations is often key/critical for the inclusion of a new medicine in the basic package of care. For patients and patient organisations, these are crucial decisions. It is important to be involved in the research of new medicines, but I think involvement in HTA decisions is much more important. They determine whether medicines become available quickly, slowly or never.
The overview below shows, for a number of countries in Europe, how many of the medicines approved by the EMA in the period 2018-2021 were part of the basic package (funded) in those countries on 5 January 2023[1]. Every representative of patient organisations should have this knowledge.
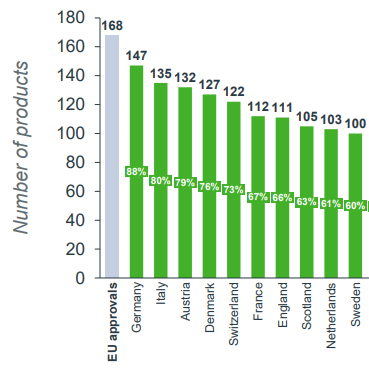
There are different reasons for the inclusion or non-inclusion of approved medicines per country. The top 10 are almost all relatively rich countries that can afford these medicines. But number 10 (Sweden) is 47 drugs behind number 1 (Germany).
Most countries that reimburse approved medicines do this in an “short” period of time. In the overview below we see the top 10 EU countries in the time between EMA approval and reimbursement decision[2].
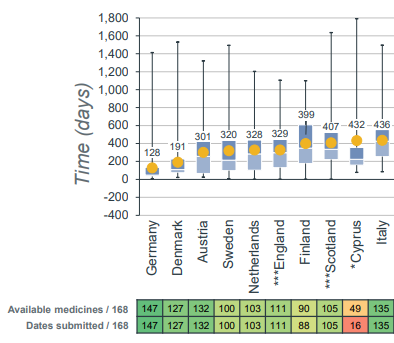
And when the decision to reimbursement is made, it takes usually three years to reach all the patients that can benefit of the new drug.
When a drug is reimbursed it will not automatically enter the daily medical practice. Recent research shows that lottery medicine exists in the Netherlands. Then, depending on the hospital where you are being treated, you may or may not get the medication you are entitled to according to professional protocol.
The question is how patient representatives could influence decision-making within HTA organisations?
Can you describe the challenges and opportunities of being involved in HTA projects?
HTA organisations calculate the cost-effectiveness of a new drug by linking the difference in price to the difference in effectiveness with an existing therapy. That is the golden standard in HTA organisations.
Crucial here is the comparator. Which medicine do you use as an HTA organisation compared to the cost-effectiveness? That comparator determines the outcome.
HTA organisations usually work with the suggested price list even though they know in advance that this price won’t be paid in practice. Worldwide reports shows that the price which countries pay for a new medicine is usually 25% – 45% lower than the suggested price list.
Finally, the cost-effectiveness is then compared to what a healthy year of life is worth in that country. If the new remedy rises above that, there is reason to start price negotiations.
The comparator, the suggested retail price and the price of life are relatively subjective and politically determined quantities in the HTA process. Patient representatives find their starting points there to exert influence on the outcome of the decision-making process.
What does it mean to be a EUPATI Dutch National Fellow for you?
I became involved in the EUPATI project HTA4Patients due to the fact that Health Technology Assessment of cancer medicines will soon become a European matter. The tables in paragraph 5 show large differences in Europe between countries that quickly make almost all medicines available to their patients and those that do not. This motivates me to think mainly European.
Through articles in Dutch newspapers and via LinkedIn, I try to draw attention to the problem of (cancer)drug inequality in Europe.
Equal access to innovative medicines is highly important for patients and their families. Spending on medicines is only seen as a cost and not as an investment. The use of quality medicines often saves other hospital expenses. The U.S. Congressional Budget Committee expects a 0.2% reduction in hospital spending with each 1% increase in the drug budget [3]. If, according to this recommendation, we spend 10 million more on medicines, 8.5 million will be saved on other hospital expenses.
In 2020, the preliminary results of a large international study, created by the National Institutes of Health in the US, were published in which a drug treatment with lifestyle advice was compared with cardio-surgical intervention and the placement of stents to limit heart attacks and mortality. “For those with angina, our results show it is just as safe to begin treating with medication and lifestyle change, and then if symptoms persist, discuss invasive treatment options”[4]. Adjusting policies in favour of medication therapy will reduce the burden on, in this case, costly cardiology and cardiosurgical hospital capacity.
Based on my experiences so far, I am working on a project to enable cancer patients in Europe to exchange knowledge and experiences with each other, in their own language. Through artificial intelligence, the written and oral texts are translated directly into the language of the other.
Talking about your experiences as a patient is best done in your own language. When patients hear that from another country of a particular drug given there, they will get information about that drug and whether it is available in their own country. One of the worst things about the big differences in access to medicine is that as a patient you don’t know that there is a medicine, but it is not available in your country or your hospital.
[1],[2] MedicalOffsets_One-col.fm (cbo.gov)
[3] https://www.nih.gov/news-events/news-releases/nih-funded-studies-show-stents-surgery-no-better-medication-lifestyle-changes-reducing-cardiac-events
[4] https://efpia.eu/media/s4qf1eqo/efpia-patient-wait-indicator_final-report-2023.pdf
Date posted: September 26, 2023
Categories: Uncategorized



