Sustainability Pillars
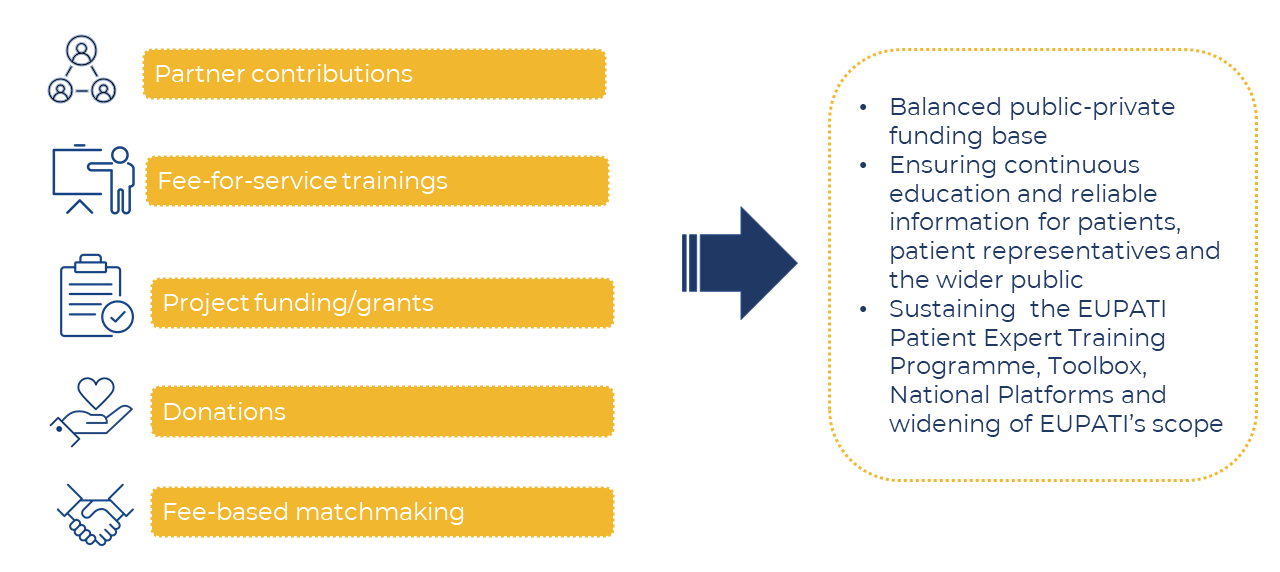
Partner contributions
The ‘Co-financing’ of EUPATI provided by the private and public partners can take the form of both, ‘in-kind’ contributions, or direct financial contributions and can be provided on the central level (EUPATI International) as well as at the national level (EUPATI National Platforms).
Fee-for-service trainings
Seeking to expand the scope of EUPATI to training for all stakeholders – including pharmaceutical industry, other health-related industry, academia, regulators and HTA bodies – EUPATI provides fee-for-service training/courses about patient engagement. The target groups are professionals working in a) patient relations/engagement management and b) in operational roles looking to engage patients.
Projects funding/grants
EUPATI participates regularly in calls for projects by e.g. IMI/IHI, the European Commission, EIT Health and other funding bodies, and has several ongoing projects. EUPATI’s role in these projects focuses on patient education and training.
Fundraising
Fundraising is an additional pillar to generate revenue with a manageable investment. This includes general fundraising and activity-based crowdfunding.
Fee-based matchmaking
Via it’s matchmaking tool EUPATIConnect, EUPATI facilitates collaborative opportunities between academic research institutions, industry partners and EUPATI Fellows, other patients and patient organisations who wish to be involved in medicines R&D.
Annual Reports
Showcasing the progress, highlights and achievements of the previous years as well as the commitment of our partners.

2023
EUPATI reached a record-wide audience in 2023. The EUPATI Toolbox reached 1 million new users (7 million since its launch) and the Open Classroom was accessed by over 160,000 new learners. EUPATI’s Patient Expert Training Programme had the highest number of registrations of all time and 81 new Fellows graduated, which is the largest cohort since the launch of the Programme in 2015 (bringing the total number of Fellows to 330+).
These figures demonstrate the important and growing need for patient-friendly information & educational resources on medicines R&D and patients’ role in this process – and the EUPATI Impact Study shows that training makes a difference in how patients and patient representatives engage with other stakeholders.
Access the full report here. Access the Financial Report including the Auditor’s remarks here and the official report for Dutch public benefit institutions (ANBI) can be found here.

2022
2022 celebrated EUPATI’s 10th anniversary, the rollout of EUPATIConnect matchmaking tool and more EUPATI Fellows graduated resulting in 254 patient experts. A new training module on Medical Devices on the Open Classroom was launched as well as a new type of patient engagement training, EUPATI Essentials piloted. The EUPATI National Platforms network continued to expand and close connections with key collaborators within the patient engagement landscape were also re-established.
Access the Financial Report including the Auditor’s remarks here and the official report for Dutch public benefit institutions (ANBI) here.

2021
The EUPATI Partnership, the National Platforms, Alumni, network of Experts and Collaborators were widely successful in enhancing patient education and patient engagement in medicines R&D. A new group of EUPATI Fellows graduated from the Patient Expert Training Programme, an unprecedented number of learners joined the new Open Classroom learning platform, and a growing number of professionals took part in EUPATI patient engagement trainings.
Access the Financial Report here and the official report for Dutch public benefit institutions (ANBI) here. The independent auditor’s report 2021 can be accessed here.

2020
2020 saw the establishment of a new EUPATI Foundation based in the Netherlands, as well the development of a new governance structure. Major changes were also introduced in the core activity of EUPATI, the Expert Patient Training Programme. An on-demand online learning platform, the EUPATI Open Classroom, was launched.



