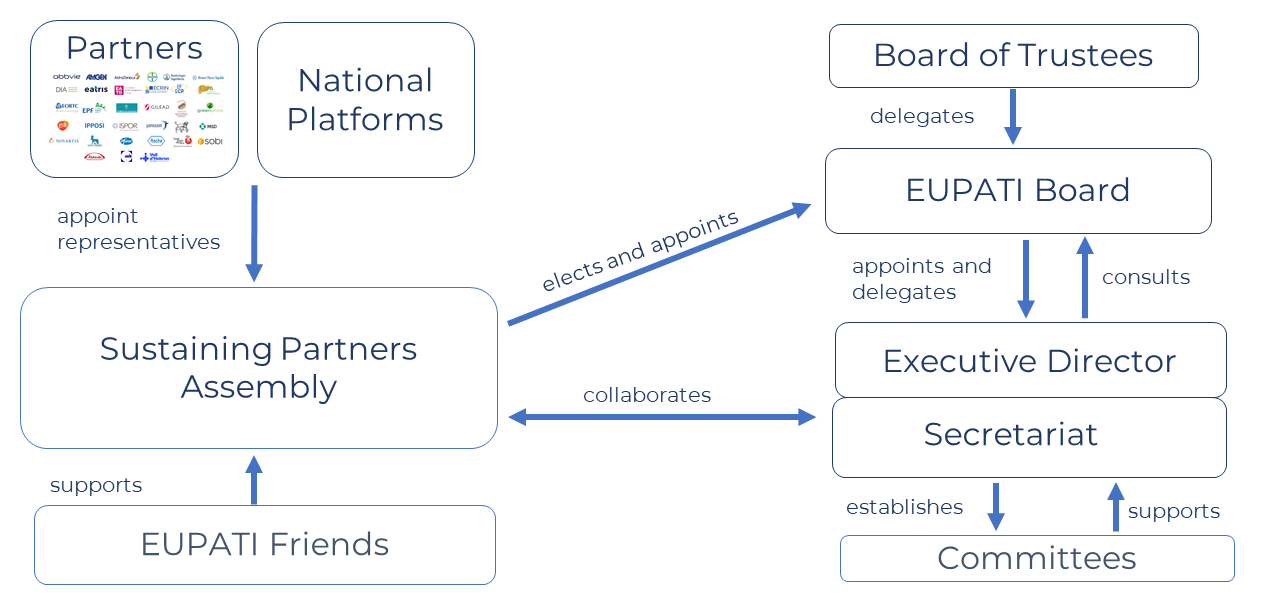
Board of Trustees
The Board of Trustees of the ‘Stichting’ EUPATI Foundation:
- Jytte Lyngvig, Denmark (Chair) – Senior consultant with assignments for WHO, previously the Senior Vice President and Managing Director for DIA Europe
- Ivett Jakab, Hungary (Vice-Chair)- Reseach Project Manager at YAGHMA, Patient advocate and health economist, former President of the European Patients’ Forum (EPF) Youth Group
- Denis Groot, Netherlands (Treasurer) – Finance Director of Foundation Lygature, a public-private partnership management organization
- Richard Bergström, Sweden – Vice President European Affairs at IQVIA, previously Covid-19 Vaccine Coordinator for the Government of Sweden, former Director General of EFPIA
- Matthias Gottwald, Germany – Independent consultant, previously Head of R&D Policy and Networking at Bayer
EUPATI Board
The EUPATI Board sets the overall strategy and takes all major strategic decisions on the direction of EUPATI. It is chaired by a patient representative proposed by the European Patients’ Forum (EPF) and co-chaired by an industry representative nominated by the industry stakeholder cluster.
The EUPATI Board is currently led by Anders Olauson from the European Patients’ Forum (EPF) as the Chair and Kay Warner from GlaxoSmithKline (GSK) as the Co-Chair. Both Anders and Kay have a long track-record in patient engagement and patient education and have contributed to EUPATI since the start.
In addition, each of the following stakeholder groups, as represented in the 4 clusters in the Sustaining Partners Assembly, can nominate 2 persons to represent them on the EUPATI Board:
– Chair: Anders Olauson (EPF)
– Co-chair: Kay Warner (GSK)
– 2 Patient Representatives: Paul Clift (EATG) and Amelia Hursey (Parkinson’s Europe)
– 2 Academic / NGO Representatives: Christine Kubiak (ECRIN) and Begonya Nafria Escalera (Sant Joan de Deu)
– 2 Industry Representatives: Donatella Decise (Sobi) and Daniel De Schryver (Johnson & Johnson Innovative Medicine)
– 2 National Platform Representatives: Sandrine Lavallé (EUPATI Luxembourg) and Jozef Glasa (EUPATI Slovakia)
Sustaining Partners Assembly
The EUPATI Sustaining Partners Assembly (SPA) consists of all EUPATI Partners committed to supporting EUPATI through in-cash or in-kind contribution. Sustaining Partners set the strategic objectives of EUPATI.
The SPA is chaired by the EUPATI Board Chair and Co-Chair and is organised in four clusters of stakeholder groups:
– Patient organisations
– Industry
– Academia & NGOs
– National Platforms
Each cluster elects representatives to the EUPATI Board which oversees the implementation of the strategic direction of EUPATI.
EUPATI invites new members of all the different clusters to join the EUPATI, please contact us for more information!
EUPATI National Platforms (ENP) Network
The National Platforms Network (ENPs) consists of representatives of each of the EUPATI National Platforms (currently 23). Each National Platform elects one individual to the so-called ENP SPA Cluster, who then select two individuals to represent ENPs in the EUPATI Board. For more information on ENPs, please see here.
Secretariat
The EUPATI Secretariat implements the decisions of the EUPATI Board, the annual work plan and is responsible for the day-to-day operations of EUPATI. It is led by the Executive Director.
The team consists of:
- Executive Director
- Partnerships & Operations Coordinator
- Training Organisation & Alumni Coordinator
- Patient Engagement Training Coordinator
- Business Development Coordinator
- Networks and Communications Coordinator
- IT Coordinator
- Open Classroom Coordinator
- EUPATIConnect & Learning Lab Coordinator
- Project Coordinator
- Intern(s)
To find our more about team, please see here.
Editorial Board
The EUPATI Editorial Board consists of 5 members representing all stakeholders. The Editorial Board reviews all new or updated content for EUPATI Toolbox and Patient Expert Course (including all related e-Learning and Face2Face course content). They guarantee that EUPATI content is well-focused, consistent, understandable, accessible and targeted. They also advice the EUPATI Secretariat about any content that needs to be updated, e.g. due to regulatory changes. The Editorial Board signs off on all EUPATI content before it is released to the public.
The members of the Editorial Board as of October 2020 are as follows:
- Jana Popova EUPATI Secretariat, Coordinator
- Magdalena Ankiersztejn-Bartczak, EATG
- Birka Lehmann, Federal Institute for Drugs and Medical Devices, Germany (BfArM)
- Wolf See, Ruhr University of Bochum
- Léa-Isabelle Proulx, Roche
The Terms of References for the Editorial Board are available to download here.
EUPATI Friends
EUPATI Friends is an informal group for individuals who have contributed to EUPATI in different capacities, but no longer have a formal role (e.g., former Partner representatives, Committee Members, Staff, Contributors, Advisors etc.) EUPATI Friends membership is by invitation and the group has no decision-making role.
The purpose is to network and stay connected, receive up to date information about the EUPATI Foundation – its vision, mission and ongoing activities, share thoughts and ideas about the future direction of EUPATI, solutions which promote the patient engagement agenda across all stakeholder groups, and act as a think tank for all the EUPATI network.
Advisory Committee
The EUPATI Advisory Committee was active during the IMI-project period until 2017, and was re-initiated in 2021. It provides advice to the EUPATI Board of Trustees, the EUPATI Board and the Secretariat on issues pertaining to regulatory, HTA, ethics and other domains. It is composed of high-level representatives with long standing experience and credibility in medicines regulation, including policies covering information to patients’ and guidance on working with patients. The current members are listed below:
- Chairperson: David Haerry, Patvocates/EATG
- Co-Chairperson: Wolf See, Ruhr University Bochum
Regulatory arm:
- Birka Lehmann, ex BfArM – Lead
- Gabriela Zenhäusern, Swissmedic
- Heather Rogers, MHRA
- Daniel O’Connor, MHRA
- Fokaline Vroom, MEB
- Leon Bongers, MEB
- Juan Estévez Álamo, AEMPS
- Christa Wirthumer-Hoche, AGES
- Katharina Prenner, AGES
HTA arm:
- Neil Bertelsen, independent – Lead
- Karen Facey, independent
Ethics arm:
- Ingrid Klingmann, EFGCP – Lead
- Hugh Davies, independent
- Uta Wernke, DLR
Future topics arm:
- Nicola Bedlington, EPF – Lead
- Jan Geissler, Patvocates
- Matthias Gottwald, Independent
- Tony Hoos, PFMD
The Terms of References for the Advisory Committee are available to download here.
Patient Expert Training Committee
Members of the multi-stakeholder EUPATI Patient Expert Training Committee contribute to the conception, development, and implementation of in-person and live streaming events linked EUPATI Patient Expert Training Programme. The composition of the committee changes regularly. The current members are listed below:
- Chair: Ingrid Klingmann, European Forum for Good Clinical Practice (EFGCP)
- Co-Chair: Ingrid Heyne, EUPATI Training Organisation and Alumni Coordinator
- Vasiliki – Rafaela Vakouftsi, EUPATI Fellow
- Barry McGrath, EUPATI Fellow
- Menia Koukougianni, EUPATI Fellow
- Nathalie Deldime, EUPATI Fellow
- Cristin Lind, ENP Sweden
- Bruno Gago, University of Aveiro, ENP Portugal
- Birka Lehmann, Senior Expert Drug Regulatory Affairs, lecturer University of Bonn
- Victoria Siegrist, Roche
- Wafae Iraqi, Janssen
- Donatella Decise, Sobi
- Sylvia Herget, CSLBerhing
- Tamás Bereczky, EATG
- Lenka Souckova, Ecrin
- Jana Popova, EUPATI Patient Engagement Training Coordinator
- Maria Dutarte, EUPATI Executive Director
- Laia Bisbal, EUPATI Open Classroom Coordinator
The Terms of References for the Patient Expert Training Committee are available to download here.
EUPATI Foundation Official Documents
Statutes
Proof of ANBI (non-profit status)
English translation:
“As from August 11 2020 we designate your institution as a public benefit institution (algemeen nut beogende instelling – ANBI).
You applied with a request to be classified as a public benefit institution. On the basis of the information provided by you it is evidenced that we can designate your institution as such.
This decision applies for an indefinite period of time. We can verify at a later stage whether your institution still meets the conditions.
[-] You are receiving this decision on behalf of the inspector.”
English translation of ANBI status
As from August 11 2020 we designate your institution as a public benefit institution (algemeen nut beogende instelling – ANBI).
You had applied with a request to be classified as a public benefit institution. On the basis of the information provided by you it is evidenced that we can designate your institution as such.
This decision applies for an indefinite period of time. We can verify at a later stage whether your institution still meets the conditions.
Are there any changes that could have an impact on the ANBI-status? You must report these changes in writing to the ANBI-team.
You are receiving this decision on behalf of the inspector.
